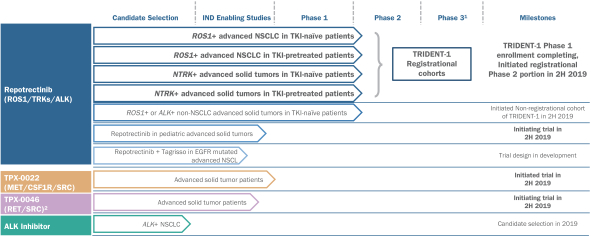
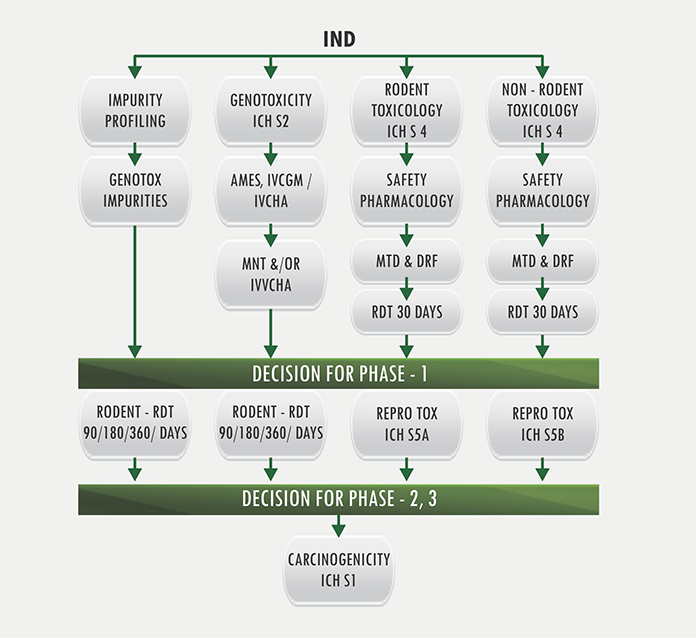
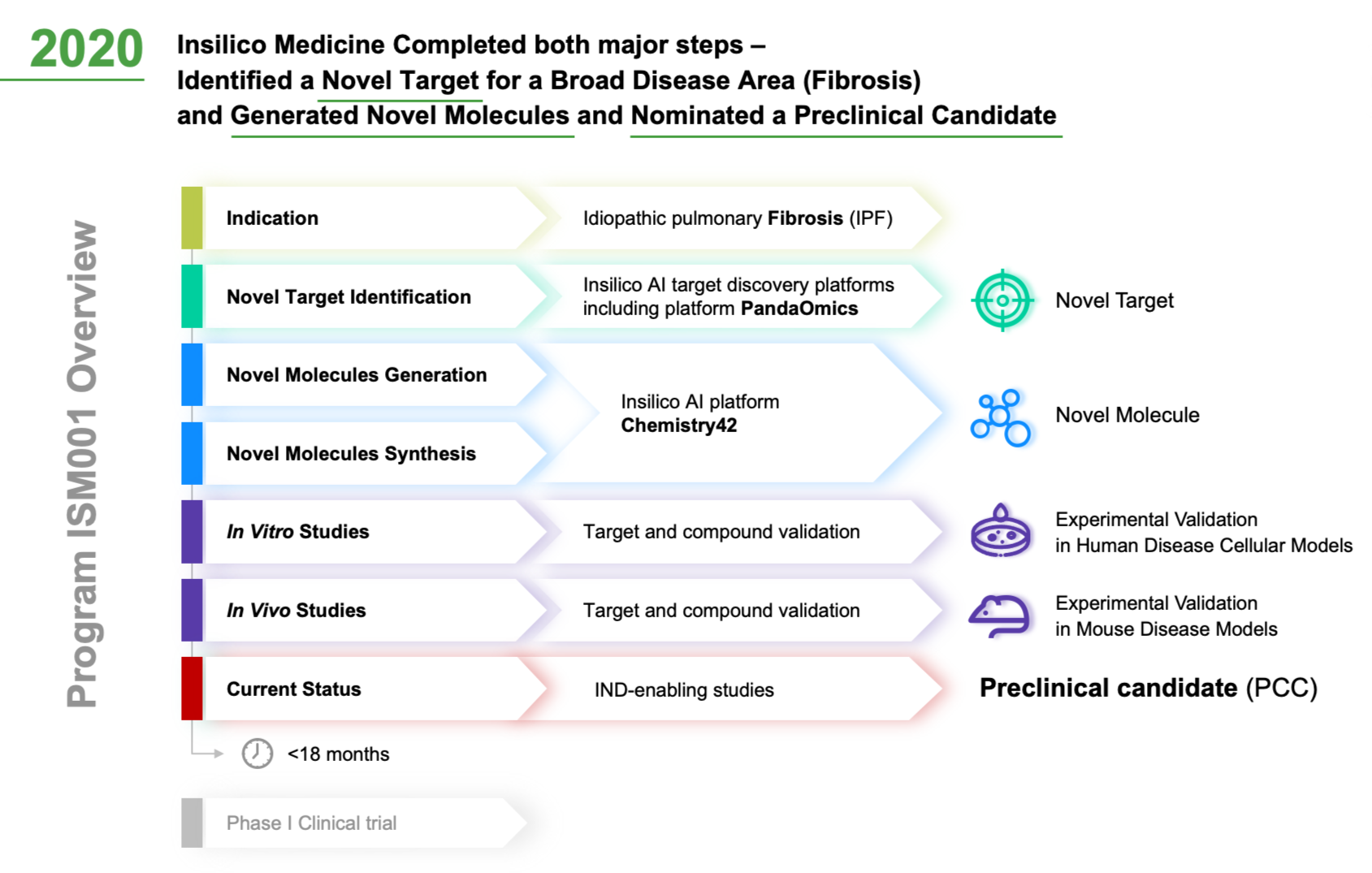
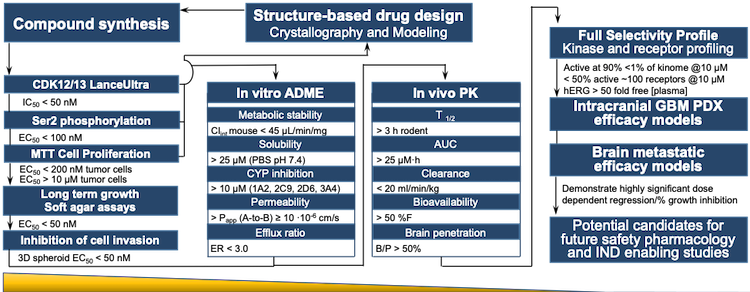
The alignment of major PK/PD related studies with the decision points... | Download Scientific Diagram
PepGen Announces IND-Enabling Preclinical Data Supporting Progression of PGN-EDODM1 into Clinical Studies | PepGen

Verve Therapeutics on X: "Today we are excited to announce anticipated 2022 milestones including timing of first HeFH patient treated with VERVE-101 & initiation of IND-enabling studies for our ANGPTL3 program. Read
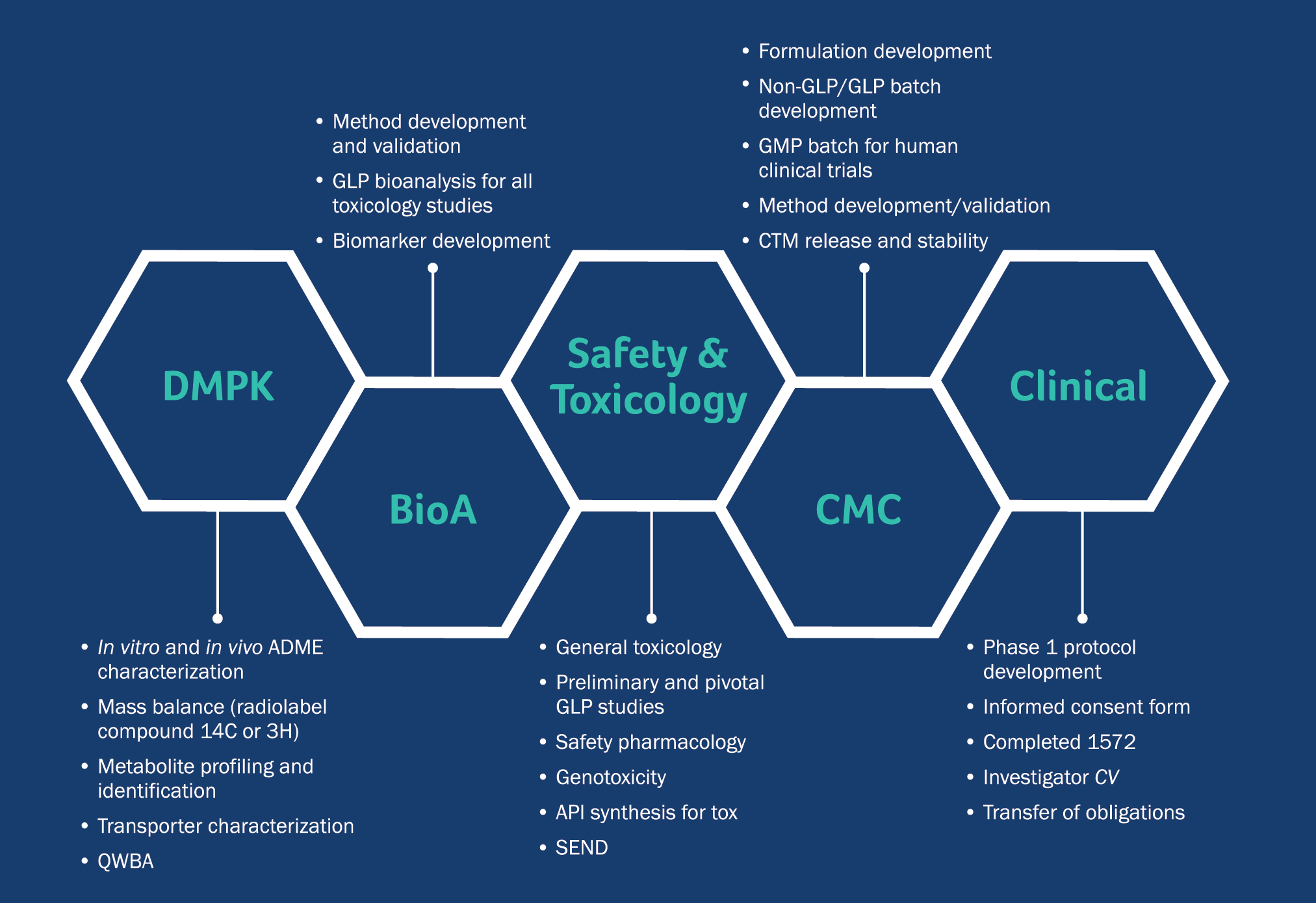
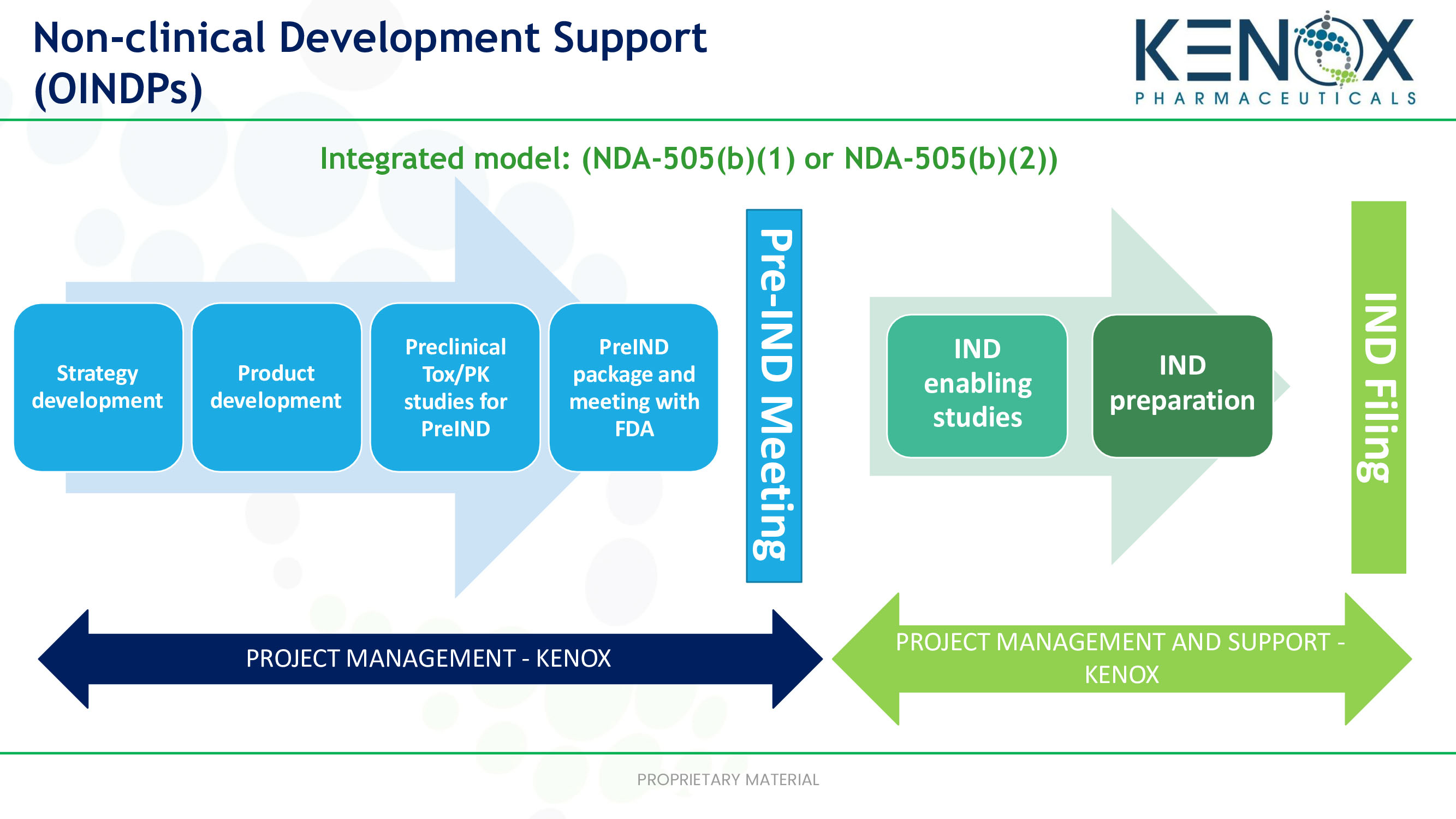
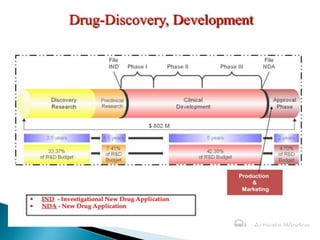
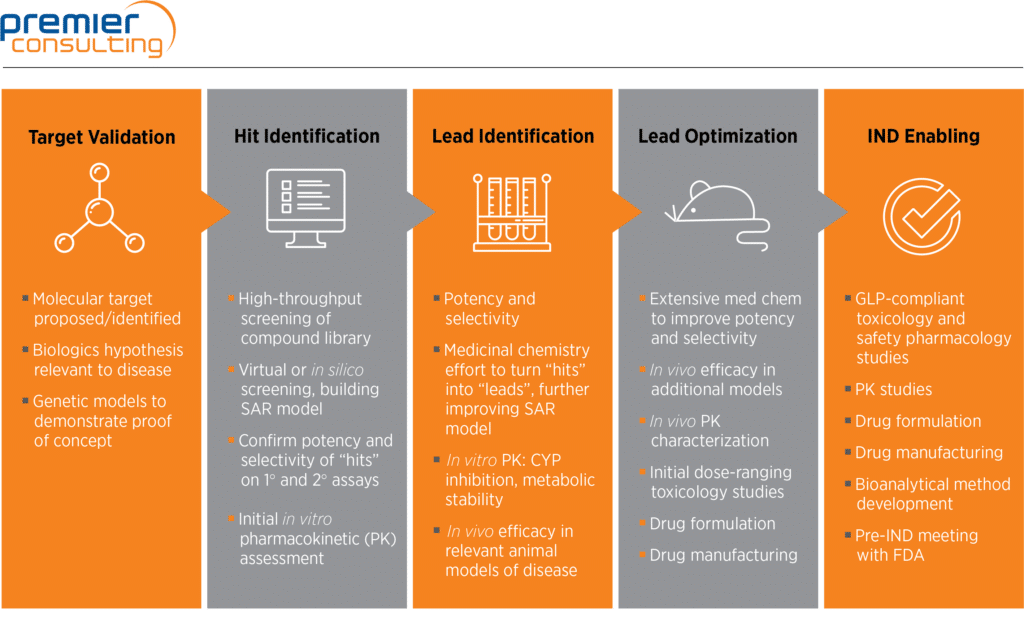
Preclinical pharmacology in IND-enabling studies and clinical pharmacology in clinical protocol development